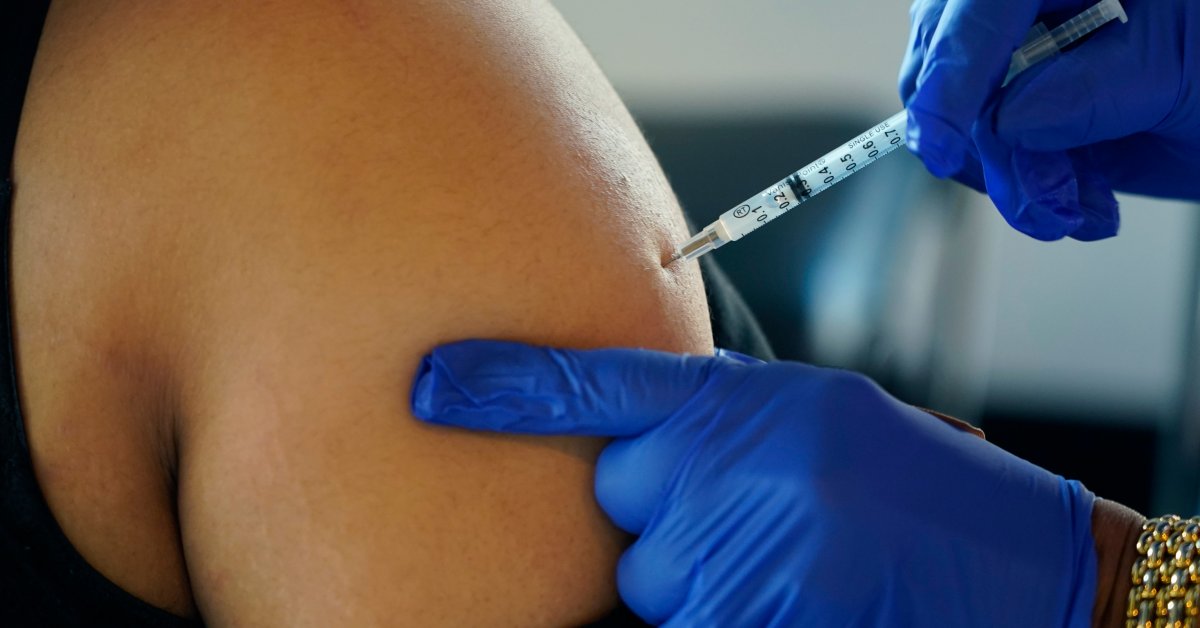
The U.S. on Wednesday licensed up-to-date COVID-19 boosters for young children as youthful as 5, trying to get to extend protection ahead of an predicted winter wave.
Tweaked boosters rolled out for People 12 and older past thirty day period, doses modified to goal today’s most common and contagious Omicron relative. Although there was not a major rush, federal wellness officials are urging that individuals seek the additional safety ahead of holiday break gatherings.
Now the Foods and Drug Administration has given a eco-friendly gentle for elementary college-age young ones to get the up to date booster doses, too—one built by Pfizer for 5- to 11-calendar year-olds, and a model from rival Moderna for all those as young as 6.
There’s 1 a lot more stage right before dad and mom can provide their kids in for the new shot: The Centers for Disease Command and Avoidance, which endorses how vaccines are used, have to indication off.
Us residents may be drained of repeated phone calls to get boosted against COVID-19, but authorities say the updated pictures have an advantage: They consist of half the recipe that targeted the primary coronavirus pressure and fifty percent safety against the dominant BA.4 and BA.5 Omicron versions.
Go through Additional: What Occurs If I Get COVID-19 and the Flu at the Similar Time?
These blend or “bivalent” boosters are built to broaden immune defenses so that people today are improved guarded towards severe disease whether they experience an Omicron relative in the coming months—or a unique mutant that is more like the unique virus.
“We want to have the best of both equally worlds,” Pfizer’s Dr. Bill Gruber, a pediatrician, advised The Associated Press. He hopes the up to date pictures will “re-energize curiosity in guarding young children for the winter season.”
The up to date boosters are “extremely important” for maintaining little ones healthy and in college, claimed Dr. Jason Newland, a pediatric infectious condition specialist at Washington College in St. Louis.
Parents really should know “there is no concern from the security viewpoint with the bivalent vaccines, regardless of whether Moderna or Pfizer,” Newland additional.
Only people today who’ve gotten their original vaccinations—with any of the primary-method versions—qualify for an updated booster. That suggests about three-fourths of Us residents 12 and more mature are eligible. As of previous weekend, only at the very least 13 million had gotten an up-to-date booster, White Residence COVID-19 coordinator Dr. Ashish Jha believed Tuesday.
To pediatricians’ chagrin, having small children their initially vaccinations has been harder. Significantly less than a 3rd of 5- to 11-12 months-olds have had their two principal doses and therefore would qualify for the new booster.
This age team will get kid-dimensions doses of the updated booster—and they can obtain it at minimum two months right after their very last dose, no matter whether that was a major vaccination or an previously booster, the Fda mentioned.
Pfizer reported it could ship up to 6 million kid-sized doses within a 7 days of authorization, in addition to ongoing adult-dose shipments.
Until now, Moderna’s up-to-date booster was cleared only for older people. Wednesday’s Fda action licensed the booster for teenagers as effectively as children as youthful as age 6.
As for even young tots, initially vaccinations didn’t open for the underneath-5 age group right until mid-June—and it will be various far more months before regulators come to a decision if they’ll also have to have a booster applying the current recipe.
Particularly how much safety does an updated COVID-19 booster shot give? That is tricky to know. Pfizer and Moderna are beginning experiments in young small children.
But the Food and drug administration cleared the COVID-19 booster tweaks without having necessitating human exam results—just like it approves yearly modifications to flu vaccines. That’s partly for the reason that both of those firms now had analyzed experimental shots tweaked to focus on prior COVID-19 variants, together with an earlier Omicron version, and observed they properly revved up virus-fighting antibodies.
“It’s obviously a much better vaccine, an significant update from what we experienced ahead of,” Jha claimed previously this 7 days.
Jha urged older people to get their up-to-date shot in October—like they get flu vaccinations—or at the very least well in advance of holiday break gatherings with large-hazard family and mates. Individuals who’ve lately had COVID-19 still will need the booster but can hold out about 3 months, he additional.
Far more Need to-Examine Tales From TIME





More Stories
Clover Health Stock Is No Longer a Meme Stock
Topical acne treatments: what’s new?
the link between skin health and mental health